Authors:
Yuan Fang, Ravi Kumar
MIT Supply Chain Management Program
37
Summary:
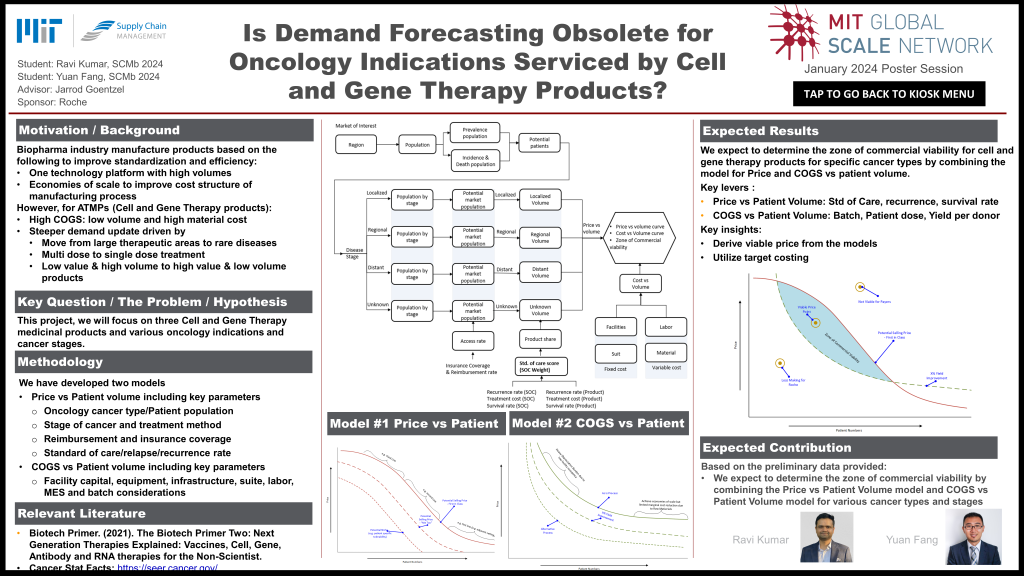
Drug development typically requires significant upfront R&D cost; however, for Advanced Therapy Medicinal Products (ATMP) such as cell and gene therapy products for oncology, the cost of goods and manufacturing costs (COGS) become a significant consideration for commercial viability. We have developed a model to output the Price/Patient Number taking into account key parameters such as oncology cancer type, patient population, stage of cancer and treatment method, reimbursement and insurance coverage, standard of care and relapse/recurrence rate. We expect to determine the zone of commercial viability for cell and gene therapy products for specific cancer types by combining the model for Price/Patient Number and Cost/Price curves.


Bravo